BIT Professor make progress with the new reversible Al3+ storage mechanism
Translator: News Agency of BIT, Chen Xisheng
Editor: News Center of BIT, Zhao Jie
¡¡¡¡
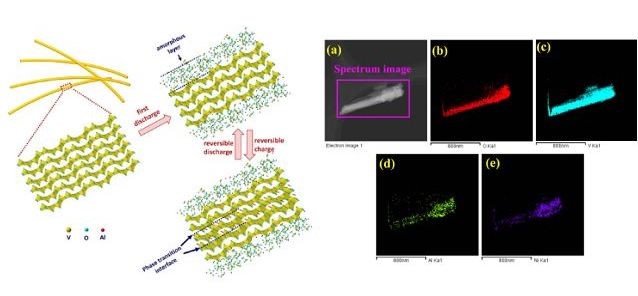
Figure 1. Schematic diagram of Al3+ electrochemical insertion/extraction in crystallized V2O5 nanowire
as well as TEM image and element mappings of a single fully discharged nanowire
¡¡¡¡
¡¡¡¡Recently, the world-renowned academic journal Energy Storage Materials (2017, 6: 9-17) published the latest achievement of the research team of Professor Wu Feng, School of Material Science and Engineering, Beijing Institute of Technology (BIT), in the new reversible Al3+ storage mechanism. The team verified for the first time that Al3+ can be inserted into the metal oxide and stored reversibly through intercalation and a phase-transition reaction, which has important implications for the development of a high-performance rechargeable battery mechanism.
¡¡¡¡
¡¡¡¡Rechargeable battery, as a high efficient and recyclable method for energy transformation and storage, is an important technological approach to comprehensively mitigate the problems of energy, resource, and environment. It has become a key part of some important applications, such as photovoltaic energy storage, electric vehicle, storage load station, and uninterruptible power supply. The rechargeable battery is also the main working power supply of current portable electric devices, ranking in the fields of major support and preferred development in main developed countries. Especially, advanced rechargeable batteries with high energy density and high power density are the focus of international cutting-edge research. How to substantially heighten the energy density and power density of rechargeable batteries and better solve the problems of battery security, further realization of the low-cost demand, and resource regeneration are posing a new challenge to subjects like material, energy, and chemistry.
¡¡¡¡
¡¡¡¡Professor Wu Feng has been working on the research in battery field for a long time. Since 2002, he has hosted three 975 projects concerning namely the Basic Research of Green Secondary Batteries (2002-2008), the Basic Research of New High-Performance Secondary Batteries and Relative Energy Materials (2009-2013), and the Basic Research of New High-Performance Secondary Batteries (2015-2019) as chief scientist of 975 Project for three consistent periods. Together with the research team he leads, Professor Wu has progressively postulated and developed the theory of multi-electron reaction. Furthermore, they have developed several typical multi-electron electrode materials, like ferrate compounds, metal borides, composite sulfur electrodes, and metal fluorides, and constructed some new mechanisms of multi-electron reaction based on it.
¡¡¡¡
¡¡¡¡According to the theory, in the electrochemical reaction, metallic aluminum can conduct trivalent reaction, and theoretically speaking, its specific mass capacity ranks only second to metallic lithium among all the metallic elements, and its specific volumetric capacity is highest among the existing metallic electrode materials, therefore, rechargeable aluminum battery based on metallic aluminum electrode has high potential in the new mechanism of high-performance rechargeable batteries. The team of Professor Wu Feng demonstrated for the first time that Al3+ can be inserted into the metal oxide and stored reversibly through intercalation and a phase-transition reaction (as shown in Figure 1), laying important foundation for more comprehensive researches into the multi-electron reaction mechanism of rechargeable aluminum batteries as well as the development of new multi-electron battery mechanism.
¡¡¡¡
¡¡¡¡The aforementioned research works provide a theoretical basis for the further development, both theoretically and experimentally, of high-performance rechargeable battery mechanism. As developing multi-electron mechanism is the best way to achieve the geometric growth of the energy density of batteries, Professor Wu Feng and his research team are still constantly pursuing and exploring the new multi-electron reaction rechargeable battery mechanism and its bases and applications.
¡¡¡¡

