BIT¡¯s Research into Doping Semiconductors NC-SI and Energy Chemistry Application Makes Progress
Translator:News Agency of BIT You Yilin
Editor: News Center of BIT Zhao Jie
¡¡¡¡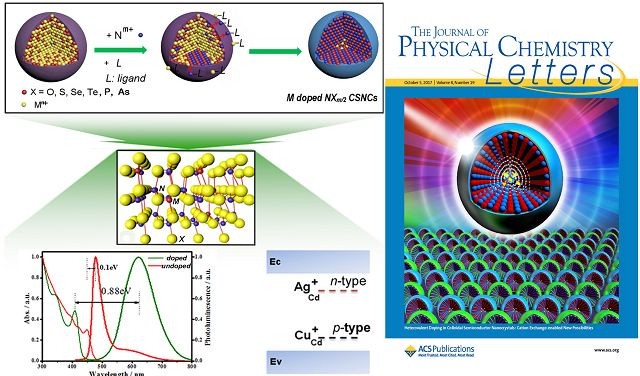
¡¡¡¡Journal of Physical Chemistry Letters magazine invited Beijing Institute of Technology (BIT)¡¯s research team to write about II-VI and III-V semiconductor nano-structure doping substitute for Perspective Cover Paper.
¡¡¡¡Semiconductors are widely applied in Optronics products because impurity can be implanted into the lattice to change its electrical property so that it¡¯ll be possible to control the spectrum, electricity and magnetism property of the semiconductor nanocrystal, and achieve the application of the new type of optoelectronic device, including high-efficient luminescent devices, solar cell and spin-electron device. And the doping problem must be solved in order to make the semiconductor nanocrystal¡¯s widespread use come true.
¡¡¡¡Zhang Jiatao¡¯s team from School of Materials Science and Engineering of BIT, has spent the last three years doing the research on getting the mixed ion doped in the II-VI semiconductor nanocrystals¡¯ depth position. They used the ion exchange reaction between amorphous semiconductor nano-particles and Main semiconductor kation, caused by phosphine ligand like TBP and PPh3, to regulate and control the thermodynamics and kinetics processing in the reaction. They finally came to the result successfully, that Ag+ and Cu+ ion can be doped in semiconductor nanostructures (quantum dot, nanosheet and 2D thin film) like CdS£¬CdSe, and CdSSe, in depth with the concentrationis under control. On one hand, this makes semiconductor quantum dot to give out mixed light steadily and efficiently (pure quantum product rate can be over 50% and remains steadily for over a year), successfully avoiding the unsteady glowing caused by self-cleaning. On the other hand, by using Ag+ and Cu+ doping, p-type and n-type II-VI quantum dot can be easily prepared and mixed in energy level regulation. This type of shining has more Stroke shift (over 0.7 eV). And by using normal position MMA part exchange, nanotechnology crystal inclusion was made to scatter evenly in the organic glasses at a centimeter level, so that the shift is enlarged to more than 0.85 eV and the Luminescence Solar Concentrator (LSC) function is improved. These results have been published successively on the top magazines SCI ¡°Angew. Chem¡± (Angew. Chem. Int. Ed. 2015, 54£¬3683-3687£©, ¡°Adv. Mater.¡± £¨Adv. Mater. 2015, 27£¬2753-2761£©¡°NPG Asian Mater¡± ( NPG Asian Mater.(2015)7,e152;doi:10.1038/am.2014.120£©and ¡°J. Phys. Chem.¡± (J. Phys. Chem. C 2017, 121, 6152?6159).
¡¡¡¡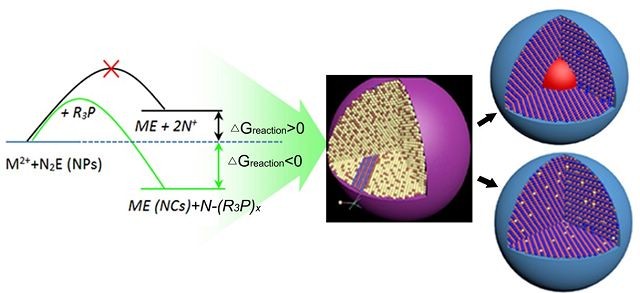
¡¡¡¡Angew. Chem. Int. Ed. magazine published new theories on preparing mixed nanocrystalline by Zhang Jiatao¡¯s team.
¡¡¡¡
¡¡¡¡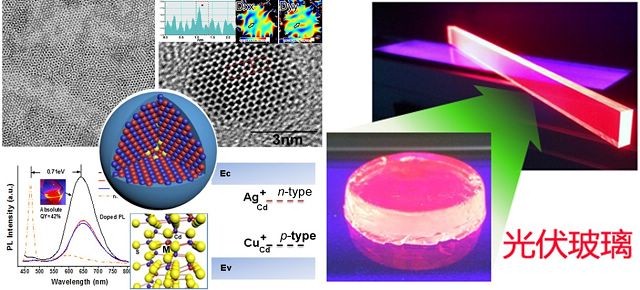
¡¡¡¡Adv. Mater. Magazine published Zhang Jiatao¡¯s team¡¯s research into new property, like preparing mixed nanocrystalline glow and regulating energy level.
¡¡¡¡
¡¡¡¡In view of the originality in the team¡¯s research progress, ¡°Journal of Physical Chemistry Letters¡± magazine invited Professor Zhang Jiatao to write an perspective essay under the title of ¡°Heterovalent doping in Colloidal Semiconductor Nanocrystals: Cation Exchange-Enabled New Accesses to Tuning Dopant Luminescence and Electronic Impurities¡± £¨J. Phys. Chem. Lett. 2017, 8, 4943?4953£©. The research team was also given a special report on the American Chemical Society website in the form of covers and videos.
¡¡¡¡
¡¡¡¡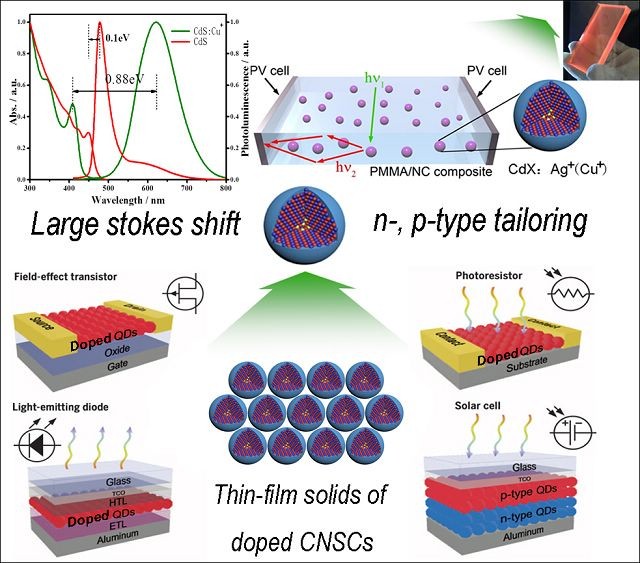
¡¡¡¡Mixed semiconductor nanocrystalline¡¯s prospect of application £¨Jiatao Zhang*, Perspective, J. Phys. Chem. Lett. 2017, 8, 4943.£© The research has received fund of NSFC¡¯s substantial project (91323301), Outstanding Youth Fund (21322105), General Program (51372025) and Main Project (51631001).
¡¡¡¡Links to releated essays:
(J. Phys. Chem. Lett.link) http://pubs.acs.org/doi/pdf/10.1021/acs.jpclett.7b00351
¡¡¡¡(Angew. Chem. Int. Ed. Link) http://onlinelibrary.wiley.com/doi/10.1002/anie.201410053/full
¡¡¡¡(Advanced Materials link) http://onlinelibrary.wiley.com/doi/10.1002/adma.201500247/full
¡¡¡¡(NPG Asia Materials link) http://www.nature.com/am/journal/v7/n1/full/am2014120a.html?foxtrotcallback="true
¡¡¡¡(J. Phys. Chem. C link) http://pubs.acs.org/doi/abs/10.1021/acs.jpcc.7b00207

